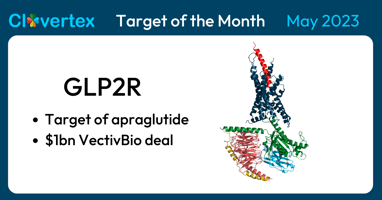
For May 2023, we're spotlighting the Glucagon-Like Peptide-2 Receptor (GLP2R) as the 'Clovertex...
Clovertex Target of the Month (09/24): PTH1R
We are pleased to announce that PTH1R (Parathyroid Hormone 1 Receptor) is Clovertex's Target of the Month for September! It's been an incredibly productive month for GPCR research, with multiple exciting developments in the field.
Septerna recently announced the initiation of its Phase 1 clinical trial for SEP-786, a novel oral PTH1R agonist targeting hypoparathyroidism, marking their transition to a clinical-stage company. In addition, Septerna has announced plans for an upcoming IPO, aiming to fund the continued development of SEP-786 and its pipeline of GPCR-targeted therapies.
Meanwhile, Superluminal Medicines closed a $120 million Series A funding round to accelerate its GPCR-targeted drug discovery efforts, and Confo Therapeutics published the first-ever cryo-EM structure of a GPCR-antibody agonist complex in Nature Communications, opening new possibilities for therapeutic interventions.
September has been a busy month for GPCR advancements, underscoring the vast potential of targeting these receptors to develop innovative therapies.

The Protein Imager rendering displays the cryo-EM structure of Human PTH1R in complex with PTH(1-34) and Gs (PDB entry 8FLQ).
About Clovertex
Clovertex is an industry leader specializing in meeting the scientific IT needs of pharmaceutical companies, with a robust focus on cloud-based or on-premises cryo-EM processing. As an Advanced Tier Services Partner with Amazon Web Services (AWS), Clovertex leverages its comprehensive expertise to architect, build, automate, and manage software applications designed to meet specific research needs. Our all-encompassing solutions are meticulously tailored to streamline the complex processes associated with pharmaceutical research, underscoring our commitment to innovation, efficiency, and customer satisfaction. By integrating state-of-the-art technology with deep scientific understanding, Clovertex paves the way in optimizing the intersection of information technology and pharmaceutical development.
Useful links
1. Septerna Announces Initiation of Phase 1 Clinical Trial with SEP-786
2. Septerna files for IPO on strength of preclinical data
3. Superluminal Medicines Gets $120M to Take GPCR Drugs to Clinic
5. Clovertex website