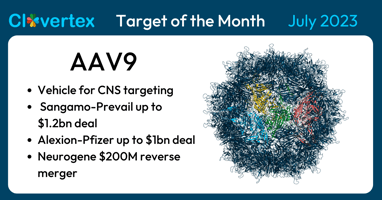
For July 2023, we focus our attention on the Adeno-Associated Virus serotype 9 (AAV9) as the...
Trikafta: Visualizing three chemical tales in one
Historically, cystic fibrosis (CF) has been among the most gruesome genetic diseases, often seen as a life-long burden of endless treatments, frequent hospitalizations, and a significantly shortened lifespan. The physical toll was often shadowed by a constant mental battle against the disease, while families who had children with CF bore the emotional weight of anxiety, uncertainty, and the omnipresent fear of loss.
The root cause of cystic fibrosis and a medical breakthrough
The F508del mutation, the most common cause of CF, affects almost 90% of CF patients globally. This mutation leads to the deletion of the 508th amino acid, phenylalanine, from the cystic fibrosis transmembrane conductance regulator (CFTR) protein. As a member of the ATP-binding cassette (ABC) transporter family, CFTR primarily functions as an ATP-gated anion channel. When F508del occurs, the absence of phenylalanine results in CFTR misfolding and degradation before CTFR can reach the cell surface and perform its function. This aberrant protein function results in a failure of chloride ion transport across cell membranes. Consequently, this leads to a build-up of thick, sticky mucus in organs, a hallmark symptom of CF. In this bleak scenario, the advent of Trikafta (Kaftrio in Europe) has been transformative. This pioneering triple combination therapy by Vertex Pharmaceuticals has significantly altered the patient's experience with the disease. By addressing this mutation directly, it sparks the hope for a majority of individuals with CF and their families to lead a life that's closer to normal.
Unveiling the chemical synergy of Trikafta by cryo-EM
The story of Trikafta's intricate chemistry is as inspiring as the hope it bestows. This drug is a combination of the folding corrector tezacaftor (VX-661), the channel potentiator ivacaftor (VX-770), and the dual-function modulator elexacaftor (VX-445). Elexacaftor's function was initially unclear, in part due to the unknown structure of F508del CFTR. Now, thanks to cryo-EM, we can understand these three interconnected stories of chemistry.
Two researchers from Rockefeller University and Howard Hughes Medical Institute in Chevy Chase have determined structures of F508del CFTR both in the absence and presence of CFTR modulators. This allowed to visualize conformational changes of CTFR when modulators are used alone or in combination providing much needed insight. For instance, elexacaftor alone only partially corrected interdomain assembly defects in F508del CFTR, but when combined with a type I corrector, it did so completely. In tandem, these correctors prevent premature degradation in the ER thus ensuring that CFTR reaches the plasma membrane. Once CFTR reaches the plasma membrane, elexacaftor secures the functioning of allosteric pore opening, thereby increasing ion conductance. Channel activity is further enhanced by ivacaftor, which stabilizes the open configuration of the pore. So this research doesn't just highlight how one drug works, which is usually exciting enough, but shows the synergistic rescue of F508del CFTR's structure and function by the three different modulators in Trikafta.
The power of 2D classification
Reflecting upon the findings of this study, three significant questions come to mind. Firstly, in light of how far we might still be from achieving gene therapy solutions, should we be intesifying the development of more Trikafta-like small molecules to combat genetic diseases?
Secondly, could we leverage the capabilities of cryo-EM to guide this exploration more rationally? By providing detailed structural information, cryo-EM could indeed facilitate the design and synthesis of molecules that effectively rescue the function of misfolded proteins.
Lastly, the use of 2D classification in this research deserves special attention. As a relatively straightforward process that can be automated, cryo-EM 2D classification has proven potent in visualizing protein folding improvements, even in 2D projection averages. It's tantalizing to imagine the potential of using this as an assay for screening compound libraries that correct protein folding.
By asking these questions and imagining their answers, we can begin to see a prominent role for cryo-EM in the futrue of genetic disease treatment. The hope is that this pioneering work will spark more investigations into the power of small molecules guided by cryo-EM, expanding our arsenal in the fight against genetic diseases.
Useful links
1. Molecular structures reveal synergistic rescue of Δ508 CFTR by Trikafta modulators.
2. Mechanism of dual pharmacological correction and potentiation
of human CFTR.
4. Derek Lowe: CFTR Correctors, Revealed