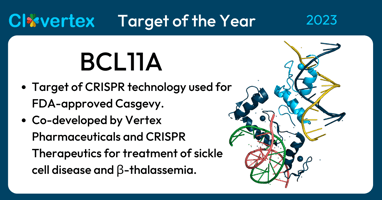
Medicine experienced a monumental moment in 2023 with the recognition of BCL11A as the 2023...
Clovertex Target of the Month (08/24): IDH1
We are excited to announce that Isocitrate Dehydrogenase 1(IDH1) has been selected as Clovertex’s Target of the Month for August. IDH1 mutations are a key driver in various cancers, particularly gliomas, and are the focus of groundbreaking new treatments such as VORANIGO® (vorasidenib), recently approved by the FDA.
This spotlight on IDH1 follows the significant news that Servier Pharmaceuticals has received FDA approval for VORANIGO, marking a major milestone as the first targeted therapy for Grade 2 IDH-mutant glioma. Gliomas are a challenging type of brain cancer, often diagnosed in younger adults, and until now, patients had limited treatment options post-surgery. VORANIGO, an inhibitor of both IDH1 and IDH2, offers glioma patients a chance to actively manage their disease with a convenient, once-daily pill.

The Protein Imager rendering displays the cryo-EM structure of isocitrate dehydrogenase (PDB entry 5K10).
About Clovertex
Clovertex is an industry leader specializing in meeting the scientific IT needs of pharmaceutical companies, with a robust focus on cloud-based or on-premises cryo-EM processing. As an Advanced Tier Services Partner with Amazon Web Services (AWS), Clovertex leverages its comprehensive expertise to architect, build, automate, and manage software applications designed to meet specific research needs. Our all-encompassing solutions are meticulously tailored to streamline the complex processes associated with pharmaceutical research, underscoring our commitment to innovation, efficiency, and customer satisfaction. By integrating state-of-the-art technology with deep scientific understanding, Clovertex paves the way in optimizing the intersection of information technology and pharmaceutical development.
Useful links
1. FDA approves vorasidenib for Grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation
2. Clovertex website